The practice of overapplying chlorine is a serious problem that is leading to negative media coverage for the aquatics industry. Many operators assume so-called breakpoint chlorination eliminates chloramines, but actually it can exacerbate the problem they are trying to solve.
A good example is the creation of nitrogen trichloride (NCl3). This is the agent considered by many researchers to be responsible for occupational asthma in lifeguards. It appears to have caused a higher incidence of asthma in children who regularly attend indoor pools, compared with those who do not, according to recent studies in the Occupational Environmental Medicine journal. And it is just one of the ammonia compounds often produced at higher levels during breakpoint chlorination in indoor facilities.
To illustrate this point further, we employed a new testing method that more accurately shows the types of disinfection byproducts formed than does traditional titrimetric analysis or DPD indicators. The method is called membrane introduction mass spectrometry, or MIMS. MIMS is highly quantitative and readily measures volatile and semivolatile, uncharged compounds in aqueous solution directly without the need for pre-concentration or other special requirements for sample preparation.
More importantly, because MIMS is a mass spectrometric technique, it can “fingerprint” different types of chlorine species formed in solution — for example, free chlorine, trihalomethanes, inorganic and organic chloramines — by molecular size analysis of fragmentation patterns. This means specific chloramine compounds can be accurately identified and their concentrations measured.
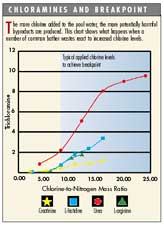
MIMS testing of water samples containing ammonia shows that at low chlorine-to-nitrogen levels, monochloramine (NH2Cl) and dichloramine (NHCl2) are formed. However, at higher ratios greater than about 8:1, which is typical of breakpoint chlorination treatment concentrations, nitrogen trichloride (or trichloramine NCl3) is the predominant chloramine byproduct. This byproduct is a nauseating, irritating gas with relatively low solubility in water. In actual pool-water environments, NCl3 is formed in solution and readily escapes into the air space above a pool. It is in part responsible for poor air quality in indoor pool environments.
It’s important to emphasize that the current practice of using breakpoint chlorination to eliminate chloramines (adding a dose of free chlorine equal to 10 times the combined chlorine concentration) almost always results in the overapplication of chlorine, high chlorine-to-nitrogen ratios and the formation of volatile byproducts such as NCl3.
Though the MIMS results with ammonia are certainly instructive, the real value of this technique is in the analysis of the chlorination of more complex organic amine contaminants that cannot be easily characterized by other methods, and are more likely to build up in pool water.
Consider the MIMS analysis of the chlorination of four amine compounds: creatinine, urea, L-arginine and L-histidine. These four amine compounds are found in pool water because they are constituents of human perspiration. Like ammonia, all of these contaminants produce NCl3, the concentration of which increases with stronger chlorine doses.
Note that at chlorine-to-nitrogen ratios of 8:1 and above, again typical of breakpoint treatment doses, significant concentrations of NCl3 are formed. Urea, the major nitrogen source compound in urine and perspiration, produces the highest levels of NCl3. Unlike ammonia, these organic amines do not undergo breakpoint, according to the Handbook of Chlorination and Alternative Disinfectants by Clifford White. The chloramines formed either persist in the water, resulting in a nuisance combined chlorine residual, or volatilize into the air space as NCl3, creating objectionable and unhealthy air quality.
For two of the amine constituents, other chlorine byproducts are detected by MIMS, in addition to NCl3. Chlorination of creatinine produces N,N-dichloromethylamine (CH3NCl2), which cause their own problems. No information could be found on the specific health effects of this compound; however, there is no question that CH3NCl2 produces an objectionable “chloramine” odor, often associated with poorly maintained indoor swimming pools. The MIMS data clearly show that even low doses (chlorine-to-nitrogen ratios of 4:1) of chlorine generate significant concentrations of this disinfection byproduct.
Chlorination of histidine produces cyanogen chloride (CNCl) and dichloroacetonitrile (CNCHCl2). Like nitrogen trichloride, cyanogen chloride is an irritant to skin, eyes and the respiratory tract. It can cause pulmonary edema (an abnormal buildup of fluid in the lungs), headaches, dizziness, confusion, nausea and vomiting. It also can affect the central nervous and cardiovascular systems, according to the National Institute for Occupational Safety and Heath. Despite the limited toxicological data on dichloroacetonitrile, the World Health Organization has been able to establish a provisional guideline for dichloroacetonitrile in drinking water of 0.02 ppm because developmental toxicity has been demonstrated in laboratory tests.
What is clear from this work is that chlorine added to pool water containing components of bather waste will form a variety of unwanted chlorinated disinfection byproducts. The greater the dose of chlorine, the greater the concentration of byproducts formed. These byproducts discussed are volatile and readily escape into the air space above a swimming pool. This can be especially problematic for confined indoor facilities, while in outdoor settings it is virtually undetectable because it is dissipated by wind. In general, these byproducts represent a serious health risk to swimmers and pool facility staff heretofore ignored.
These ammonia compounds are an unavoidable consequence of the reaction of free chlorine sanitizer and components of bather waste, and cannot be entirely prevented or eliminated. So operators must try to manage the chlorine demand and minimize the formation of unwanted byproducts — and the odors, irritation and potential health effects associated with them. A good place to start is by reducing, or even eliminating, breakpoint chlorination techniques that are targeted at reducing combined chlorine.