The choice of sanitizer can significantly impact the costs of operating a large aquatics facility. This article addresses potential costs associated with two commonly used sanitizers: calcium hypochlorite (or cal hypo) and trichloroisocyanuric acid (or trichlor).
When these chemicals are added to pool water, they clearly increase available chlorine, however they also affect pH, alkalinity and either calcium hardness or cyanuric acid levels.
Think of it this way: Every time these sanitizers are added to the water, they bring additional chemicals “inside” them as well. Sometimes these “extra” chemicals are beneficial, sometimes you will have to pay to get rid of them.
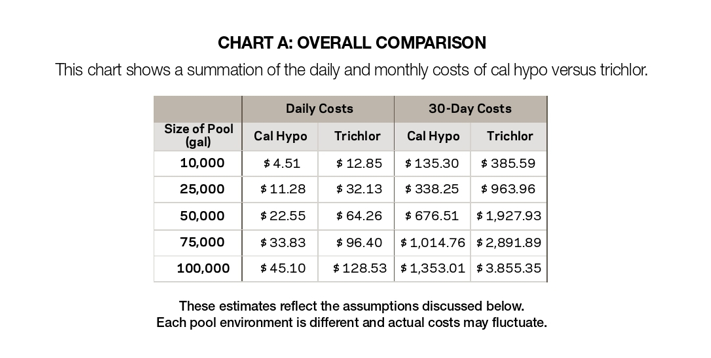
To compare the costs of using cal hypo with those of using trichlor, an estimate was made based on 2017 water and sewage rates in Fulton County, Ga., combined with 2017 chemical costs. This estimate shows that costs incurred by facilities using cal hypo might be considerably lower than those for the same facility using trichlor. Chart A shows estimated costs when adding 10 parts per million of available chlorine to a pool using the two sanitizers. An explanation of the assumptions used for the estimate follows.
Innovative Water Care
Explanation of assumptions and estimates
When estimating the costs of treating pool water, simply looking at the price per pound of sanitizer does not account for less obvious costs and may lead to false assumptions about savings.
You also must consider how each sanitizer affects the water’s balance. In addition to raising available chlorine, cal hypo boosts pH, alkalinity and calcium hardness (CH) levels.
Trichlor has the opposite effect on pH and alkalinity — reducing it. And where cal hypo affects CH, trichlor increases cyanuric acid (CYA).
As a result of sanitizer choice, a facility may need to replace some pool water to counter a rise in CH or CYA levels. This can result in higher water and sewage costs. Further, because both sanitizers affect pH and alkalinity, this typically will need to be corrected with additional chemicals, further adding to costs.
Every pool is different, and accounting for every scenario may be impossible. To estimate costs incurred by the use of these sanitizers, certain assumptions were made for this comparison. The following explanations describe the reasoning behind these assumptions.
Water usage
The amount of water consumed is a substantial component of running any aquatics facility.
As previously mentioned, cal hypo adds to calcium hardness, while trichlor adds cyanuric acid. Neither CH nor CYA are destroyed by chlorine, nor do they evaporate. This leaves draining and replacing water as the only practical way to decrease CH and CYA concentrations.
Splashout and filter backwashing reduce concentrations, but their impact changes from pool to pool, making it difficult to estimate effects on water consumption. Therefore, water usage from these activities was left out of this paper’s estimates. Nor was water consumption from evaporation factored in, since evaporation does not remove either chemical.
Cal hypo
Calcium hardness plays a vital role in water chemistry, helping protect plaster, concrete and metal objects from corrosion caused by aggressive water.
A minimum of 150 ppm CH is required for pool water, as stipulated in ANSI/APSP-11. For plaster pools, the National Plasterers Council recommends a CH of at least 200 ppm. When the water falls between 150 and 1,000 ppm of CH, the saturation index can be maintained to prevent scale and cloudy water formation, according to ANSI/APSP-11.
Adding 10 ppm of available chlorine using calcium hypochlorite will add 8 ppm CH, according to APSP. For a pool starting at 150 ppm CH, adding 10 ppm available chlorine daily with cal hypo would cause the CH in the pool water to reach 1,006 ppm in 107 days.
When water reaches 1,000 ppm CH, removing and replacing 1% will drop the CH 8 ppm, if the source water is 200 ppm CH. In a 100,000-gallon pool, the replacement amount equals 1,000 gallons. This will counter the CH added by the daily dose of cal hypo at these concentrations and rates.
Trichlor
Cyanuric acid (CYA) helps protect the available chlorine from decomposition caused by UV light.
A maximum of 100 ppm CYA is recommended by ANSI/APSP-11, while the Model Aquatic Health Code recommends a maximum of 90 ppm CYA. For indoor pools, CYA is considered unnecessary and is not recommended, according to ANSI/APSP-11.
Adding 10 ppm of available chlorine using trichlor will raise CYA by 6 ppm, according to APSP. For a pool starting at 30 ppm CYA, using trichlor to add 10 ppm of available chlorine each day would cause the CYA to reach 90 ppm in 10 days and 102 ppm in 12 days.
When CYA levels reach 90 ppm, 6.7% of the water volume must be removed and replaced to counter this daily addition of trichlor. At 100 ppm CYA, removing and replacing 6.0% of the water will counter the CYA added daily by the use of trichlor. For a 100,000-gallon pool, that amounts to 6,700 gallons and 6,000 gallons respectively.
Estimating water and sewage costs
Minimizing the costs of water and sewage presents a substantial opportunity for pool operators to save money.
Examples show that, to provide the same amount of available chlorine, sanitizing with trichlor can take six times or more added water to keep the CYA in a recommended range than the amount needed to keep the CH in a recommended range when using cal hypo.
Since this water must be drained and refilled, costs go up even more in locations where sewer rates are based on consumption. In those areas, not only would the water costs be six times higher, but sewer costs would be as well.
Water and sewage costs vary widely around the country, so calculating them can be complex. In this analysis, we used Fulton County’s 2017 water and sewage costs. Water and sewer rates scale up depending on monthly usage.
To compare apples to apples, we assumed that the source water contains 200 ppm CH; the maximum recommendations of CH or CYA had been reached, making it necessary to drain and refill; and the top tier water price had been reached by water consumption not associated with the sanitizer (water cost $0.0100 per gallon and sewage cost $0.0055 per gallon). Water loss due to evaporation, splashout and filter backwashing was excluded.
Chemical costs
In addition to changing the calcium hardness or cyanuric acid levels, these sanitizers affect the water’s pH and alkalinity.
Cal hypo contains small amounts of calcium hydroxide and calcium carbonate, resulting in slightly increased pH and total alkalinity. In theory, 10.5 ounces of cal hypo added to a 10,000-gallon pool (adding 5.1 ppm available chlorine) will increase pH by 0.009 and carbonate alkalinity by 0.29 ppm, if the starting conditions of the water were a pH of 7.5, 100 ppm carbonate alkalinity, and 1,000 ppm total dissolved solids, as stated in “Swimming Pool Water Balance — Part 2: Factors Affecting the Calcium Carbonate Saturation Index,” by J.A. Wojtowicz.
Conversely, in the same pool, 7 ounces of trichlor (adding 4.7 ppm available chlorine) will theoretically decrease pH by 0.14 and decrease total alkalinity by 3.3 ppm, under the same conditions, also according to Wojtowicz.
Muriatic acid and sodium carbonate are common choices for large commercial and municipal pools to neutralize pH changes in pool water. Although there are other methods, this paper assumes the use of muriatic acid to neutralize the increased pH caused by cal hypo, and sodium carbonate to neutralize the pH decrease cause by trichlor. A study performed by Olin Corporation found that, on average, 1.56 ounces of 32% muriatic acid neutralized the pH of 1 pound of cal hypo. In the same Olin study, 0.93 pounds of sodium carbonate was found to neutralize the pH of 1 pound of trichlor.
Combined chemical costs were determined using the above chemical usage rates for pH neutralization and the following chemical costs:
• Cal hypo: $2.30 per pound
• 32% muriatic acid: $0.055 per ounce/ $7.00 per gallon
• Trichlor: $2.16 per pound
• Sodium carbonate: $1.80 per pound
The chemical cost to counter the pH effect of 1 pound of cal hypo adds $0.09, where the chemical costs to counter the pH effect of 1 pound of trichlor adds $1.67 per pound.
The combined chemical costs per pound of cal hypo would be $2.39, and per pound of trichlor would be $3.83.
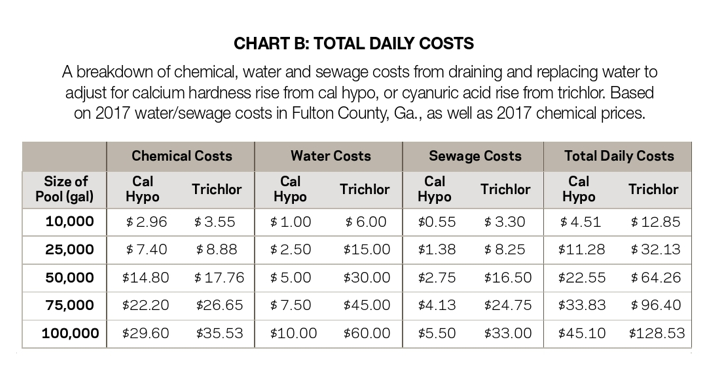
With cal hypo at 68% available chlorine, and trichlor at 90%, combined chemical costs per pound of available chlorine for cal hypo would be $3.51; for trichlor, $4.26. With 10 ppm available chlorine added daily, Chart B estimates the daily and monthly costs for chemicals, water and waste for several pool sizes.
Innovative Water Care



