For the past 16 years, I have been traveling around the United States training operators. During that time, one thing has become abundantly clear: There’s a lot of confusion about the relationship — and the difference between — oxidation reduction potential and parts per million.
Part of the problem is that many state health departments don’t regulate ORP, but they do regulate ppm. So operators tend to focus on ppm and forget about ORP. That’s a mistake.
When it comes to maintaining healthy pool water, ORP is actually more important than ppm. What’s more, relying on ppm readings alone can provide a false, and dangerous, sense of security.
To understand why, we have to start with basic water chemistry. When chlorine is added to water, a chemical reaction occurs between chlorine and the water, called hydrolysis. During hydrolysis, chlorine reacts rapidly with water to form two separate chemicals: hypochlorus acid (HOCl) and hydrochloric acid (HCl).
Of these two compounds, the important one is hypochlorus acid, or HOCl. This compound is the “active form” of chlorine, providing the needed oxidation, substitution and disinfection reactions. Without getting too technical, you want significant HOCl in the pool water for effective oxidation and disinfection.
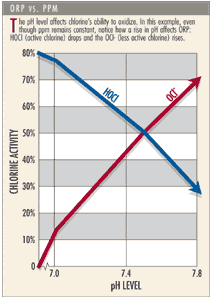
However, ppm and HOCl do not always correspond. That’s because HOCl dissociates or separates into two components, the hydrogen ion (H+) and the hypochlorite ion (OCl-). Like the hydrolysis reaction, the degree of dissociation is primarily dependent on temperature and the pH of the water. As the pH goes up (a shift to the right), the hypochlorite concentration increases. As the pH goes down (a shift to the left), the hypochlorus concentration is increased.
Therefore, while your test kit may show ample amounts of chlorine at a pH 7.8, which is within most health code standards, the HOCl may not be as effective at killing pathogens and making the water clear. At a pH 7.8, you only have 30 percent HOCl, though the ppm of chlorine remains the same. That’s why it can be misleading to think that a pool with a pH of 7.8 and “free” chlorine level of 1 to 2 ppm is a healthy swimming environment.
Most health departments don’t require an ORP minimum, but decades of research have shown that ORP is a direct measure of the sanitizer’s oxidation and disinfection strength in the water. For instance, the polio virus dies in five minutes at an ORP level of 550 millivolts (mV), but at a 650 mV ORP level, it takes only 30 seconds to kill this virus. Add another 100 mV and the kill time is approaching one second! Kill rates have been similarly established for all waterborne pathogens along the ORP rate-of-disinfection scale — even for cryptosporidium, E.coli, giardia and legionella. ORP essentially measures the effectiveness of the sanitizer in the water. The lower the ORP, the less HOCl in the water and the less effective the sanitizer.
The problem with ORP is that it can’t be measured with standard test kits. Options for determining ORP are small, inaccurate handheld devices or automated controllers.
So the next time you take your chlorine readings, make sure you check and record the ORP, too. If you don’t have a device to measure ORP, maybe it’s time to consider getting one. And remember, ORP is the true measure of safe water.