Since the early 1960s, the pool industry has been using a familiar concept called the Calcium Saturation Index to predict water’s tendency to be corrosive or scale-forming. And for just as long, it has been our friend and our nemesis. The index is fraught such misunderstanding, causing some aquatics professionals to question its application to pools. With a better understanding of the common myths and mistakes associated with the Calcium Saturation Index, pool operators will be able to apply this important concept of pool-water balance in a reliable manner.
The original Calcium Saturation Index (CSI) was derived from a theoretical concept by Dr. Wilfred Langelier, professor emeritus of civil engineering at the University of California, Berkeley. His paper detailing the model was first published in 1936. Since then, professor Langelier’s index has become widely used and studied, not only in the pool industry but also in industrial and domestic drinking water treatment as well as other applications. It is interesting to note that his work dealt with enclosed loops of municipal water piping systems and he never made mention of its use on water loops that are open to the atmosphere, such as swimming pools.
The concept of pool-water balance, as determined using the CSI, is very important to the trained operator. The numerical expression helps predict the water’s aggressiveness or scaling potential. The CSI itself is composed of five variables: pH, temperature, bicarbonate alkalinity, calcium hardness and total dissolved solids. The classic representation of the CSI is as follows:
CSI = pH + Tf + Af + Cf ? 12.1
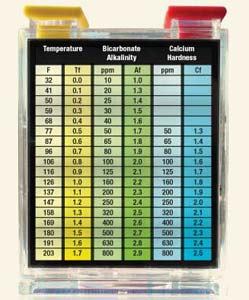
In this simplified formula, pH is inserted directly and the appropriate factors for temperature, bicarbonate (total) alkalinity and calcium hardness are taken from a table and entered into the formula (the factor for total dissolved solids is included in the constant). The resulting calculated value is the pool water’s “balance.” Negative values indicate increasingly aggressive water while positive values indicate the water has an increasing potential to scale. Water that yields a CSI of between –0.3 and +0.3 generally is considered “balanced.” The goal is to avoid highly aggressive water that will degrade the pool surface and equipment while also avoiding scaling water, which can deposit scale on pool interiors, pipes and the like.
With its readily identifiable benefits to preserving the pool structure and equipment, there are some notable flaws and fallacies with the index itself. Common errors in understanding the Calcium Saturation Index include correct terminology, what is considered “balanced,” CSI parameters, temperature factor correction and the influence of total dissolved solids.
While seemingly trivial, proper terminology is a foundation for understanding what the CSI does and does not do. First, the correct title for this concept is “Calcium Saturation Index,” not “Langelier Saturation Index,” “Langelier Index” or “Saturation Index.” This is the term professor Langelier used and the only name that is adequately descriptive of what is saturated.
Second, negative CSI values show water’s tendency to be aggressive, not corrosive. Dr. Langelier’s original work focused on predicting precipitation of calcium scale, and scholars agree that there is no correlation between the corrosion of metal and the CSI. Negative values do, however, show the water’s aggressiveness toward plaster and other nonmetallic surfaces as it seeks to dissolve calcium and reach equilibrium.
Another fallacy is that the CSI predicts scale-forming water. In fact, it does not. The CSI only predicts the water’s scaling potential. Pool water with CSI values of +0.5, even +1.0 and higher, will not precipitate scale in a well-managed pool. It takes a pH above 8 (around 8.3 to 8.4) for calcium carbonate to fall out of solution. This is actually related to the alkalinity, the buffering capacity of the water. At a typical pool pH of 7.2 to 7.6, no scale can form.
When interpreting the CSI, it’s important to know that zero is not necessarily perfectly “balanced.” A variety of factors make the wisest choice subjective for any particular pool. Some operators choose to hold a –0.1 to account for “up-scaling” heaters, but many times holding +0.3 or more, with an understanding of the Ryzner Index, is the preferred point while keeping a nice, low pH to maximize chlorine’s effectiveness.
A little-known fact is that the Calcium Saturation Index has been tested and shown to only work inside certain parameters for each variable. While most of the parameter limits exceed what is likely in a pool or spa, it should be noted that the reliability of the index is severely compromised if any value falls outside the following parameter limits:
pH: 6.5 – 9.5
Temperature: 32° – 212° F
Bicarbonate Alkalinity: 10 – 800 ppm
Calcium Hardness: 50 – 700 ppm
(up to 900 ppm)
Total Dissolved Solids: 50 – 1000
One largely unknown flaw is in the published tables. The temperature factors have been miscalculated and ever since, copied in all major pool operation texts. Table 1 shows all of the recalculated, correct factors for each variable. The only notable differences appear in the temperature column, which is only about 0.1 in the typical pool and spa range. It also should be noted that the table complies with the previously mentioned CSI parameters.
Over the years, the total dissolved solids (TDS) influence has been greatly exaggerated. The constant (12.1) includes the TDS factor for 1,000 parts per million of TDS. This is not an uncommon value in many pools. It is important to know that the CSI has only been shown to work with TDS values up to 1,000 ppm.
But, using professor Langelier’s original equation, the next tenth of negative influence would not occur until 10,000 ppm TDS, not the commonly referenced 1,000 ppm. It can safely be assumed that unless a pool is filled with seawater, the influence of TDS is negligible and can therefore be ignored altogether in most pools. Even in pools and spas using chlorine generation, commonly called saltwater systems, where the salt content is typically maintained between 3,000 and 6,000
ppm, the TDS influence is small enough to be ignored.
With all the associated problems with the Calcium Saturation Index, the question persists, “Is there a better way?” In short, the answer is “no,” but there are some other methods worth discussing. The Ryzner Stability Index (RSI) has received some deserved attention from the industry, and has been applied very successfully in some cases. The formula for the Ryzner Index is:
RSI = 2 x (12.1 ? Tf ? Af ? Cf) ? pH
The midpoint for this index is 6.5, with values below 6.0 indicating an increasing scaling potential and values well above 7.0 indicate true corrosive tendencies of the water.
While the Ryzner Index is useful for predicting water’s corrosiveness, it becomes apparent that simply maintaining a positive CSI value with high calcium hardness will yield similar results.